7
SECTION 2. PRINCIPLES OF OPERATION
MODEL TCL SECTION 2
PRINCIPLES OF OPERATION
Total chlorine by definition is the iodine pro-
duced in a sample when it is treated with
potassium iodide at a pH between 3.5 and 4.5.
Typically, acetic acid (or vinegar) is used to
adjust the pH.
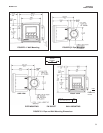
The total chlorine analyzer consists of a sam-
ple conditioning system, which injects the
reagent into the sample, and a sensor and
analyzer, which measure the amount of iodine
produced. Figure 2-1 shows the sample condi-
tioning system. The sample enters the sample
conditioning enclosure and flows to an over-
flow sampler from which the sample pump
takes suction. Excess sample drains to waste.
At the same time, the reagent pump draws
reagent, a solution of potassium iodide in vine-
gar, from the reagent carboy and injects it into
the suction side of the sample pump. The sam-
ple and reagent mix as they pass through the
pump, and total chlorine in the sample is con-
verted to the chemically equivalent amount of
iodine. The flow rates are 11 mL/min for the
sample and 0.2 mL/min for the reagent.
The treated sample next enters the flow cell. Bubbles injected into the flow cell produce turbulence, which
improves the stability of the reading. A membrane-covered amperometric sensor in the flow cell measures the
concentration of iodine. The analyzer receives the raw signal from the sensor and displays the concentration of
total chlorine. Display units are ppm (mg/L) chlorine as Cl
2
. The treated sample leaves the flow cell and drains to
waste along with the excess sample.
FIGURE 2-1. Schematic of Sample Conditioning
System and Analyzer.