THEORY OF OPERATION M102E/M501 TRS
(Addendum to M101E Manual - P/N 04740 Rev A)
nm. The SO
2
molecules absorbs some of energy from the UV light causing one of the electrons of
each of the affected molecules to move to a higher energy orbital state.
*2SOhvSO
Ia
nm2142
⎯⎯→⎯+
(Equation 9-2)
The amount of SO
2
converted to
excited SO
2
* in the sample chamber is dependent on the
average intensity of the UV light (
Ia) and not its peak intensity because the intensity of UV light
is not constant in every part of the sample chamber. Some of the photons are absorbed by the
SO
2
as the light travels through the sample gas.
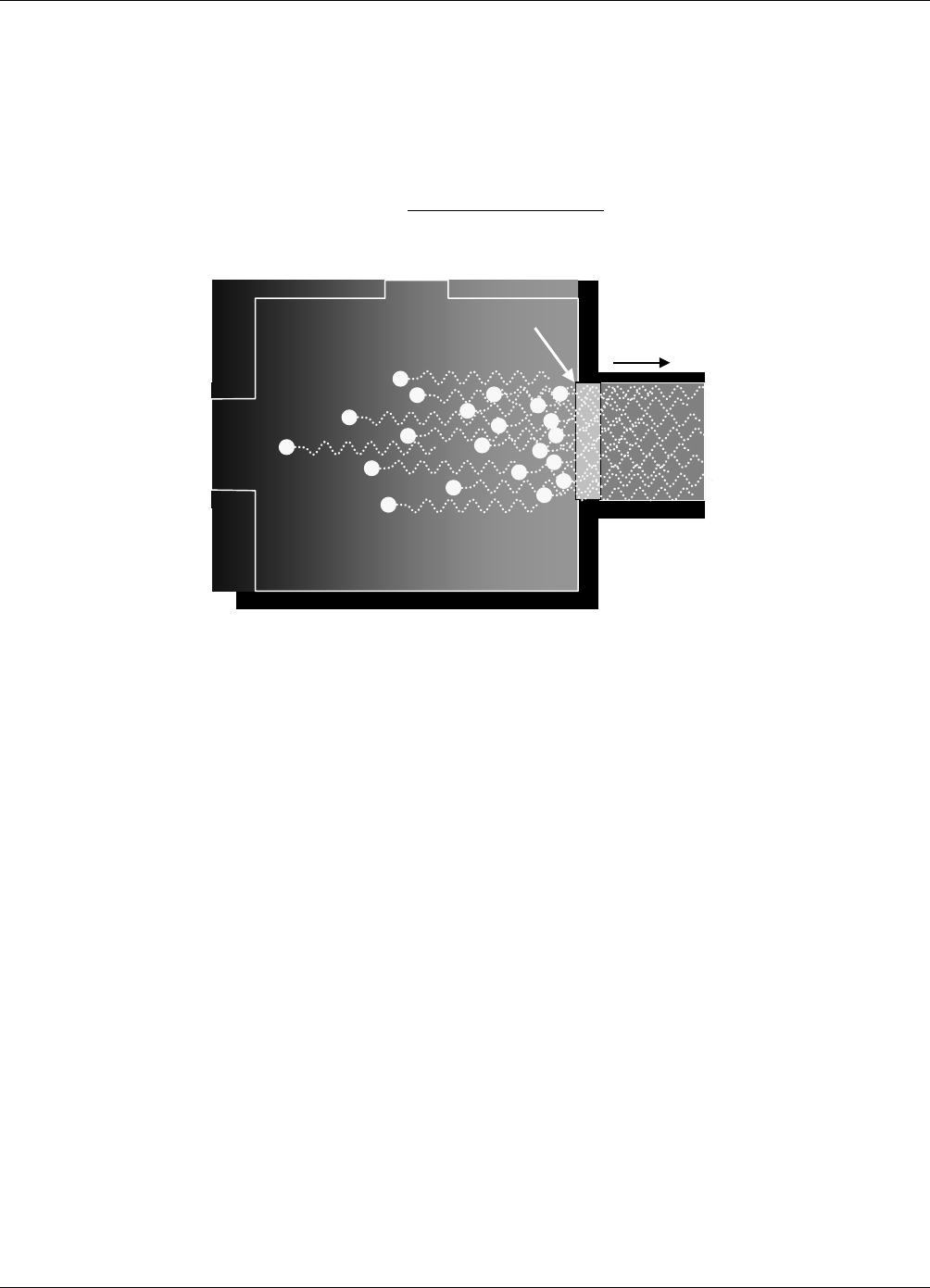
Darkened
REACTION CELL
filled with SO
2
214nm
Filter
UV
SOURCE
Figure 9-1: UV Absorption in the M102E Reaction Cell
The equation for defining the average intensity of the UV light (Ia) is:
(
)
(
)
[
]
20
SOaxexp1IIa
−
−
=
(Equation 9-3)
Where:
I
0
= Intensity of the excitation UV light.
a = The absorption coefficient of SO
2
(a constant).
SO
2
= Concentration of SO
2
in the sample chamber.
x = The distance between the UV source and the SO
2
molecule(s) being
affected (path length).
The second stage of this reaction occurs after the SO
2
reaches its excited state (SO
2
*). Because
the system will seek the lowest available stable energy state, the SO
2
* molecule quickly returns to
its ground state (Equation 10-3) by giving off the excess energy in the form of a photon (hν). The
wavelength of this fluoresced light is also in the ultraviolet band but at a longer (lower energy)
wavelength centered at 330nm.
nm33022
hvSO*SO +⎯⎯→⎯
(Equation 9-4)
44 05514 Rev A1


















