Teledyne API T703/T703U Calibrator Operation Manual Principles of Operation
211
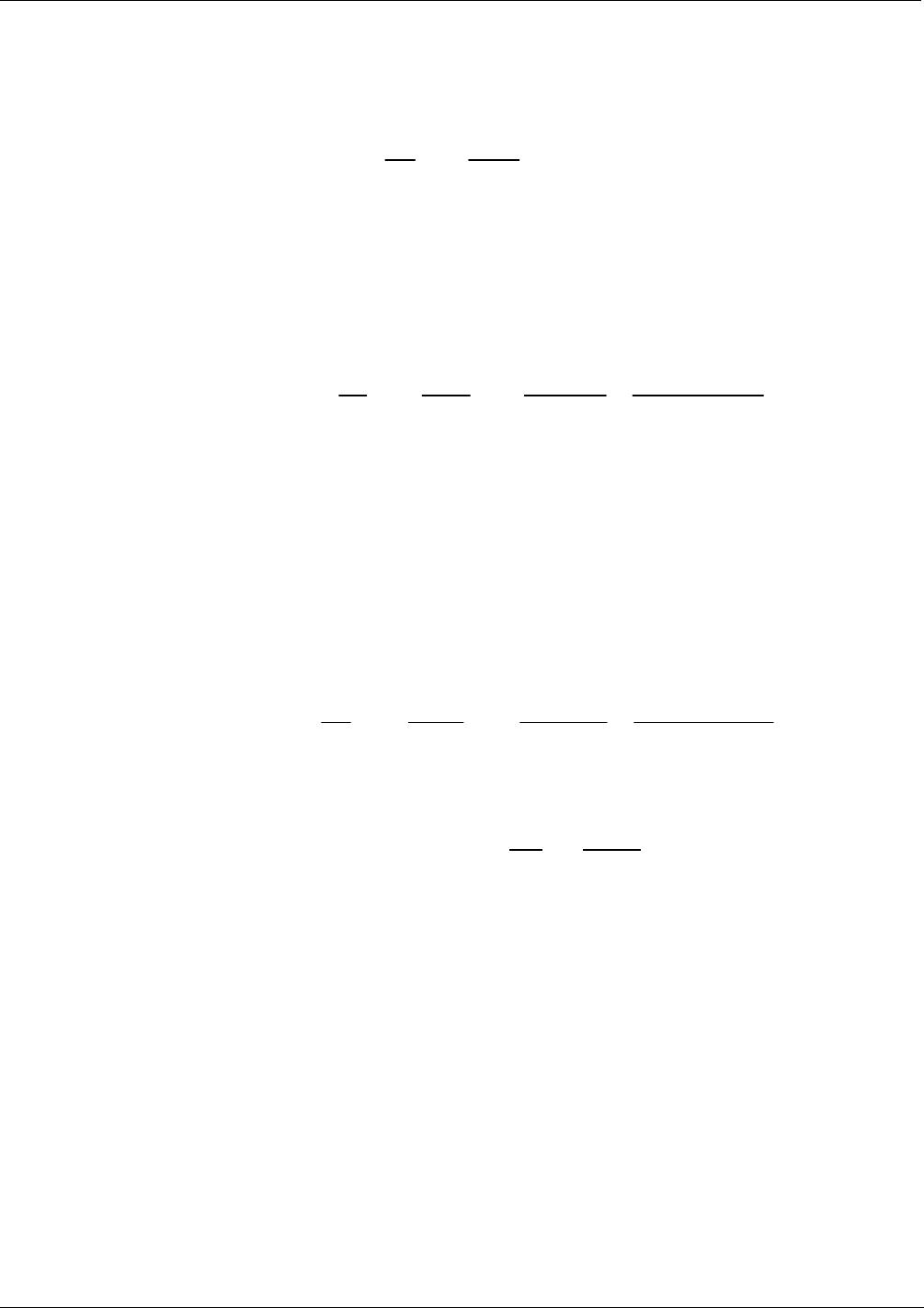
To solve this equation for C, the concentration of the absorbing Gas (in this case O
3
), the
application of a little algebra is required to rearrange the equation as follows:
Equation 9-6
LI
I
C
o
1
ln
at STP
Unfortunately, both ambient temperature and pressure influence the density of the
sample gas and therefore the number of ozone molecules present in the absorption tube
thus changing the amount of light absorbed.
In order to account for this effect the following addition is made to the equation:
Equation 9-7
Ρ
inHgΤ
LI
I
C
o
92.29
273
1
ln
Where:
T = sample ambient temperature in degrees Kelvin
P = ambient pressure in inches of mercury
Finally, to convert the result into Parts per Billion (PPB), the following change is made:
Equation 9-8
inHg
LI
I
C
o
92.29
273
10
ln
9
In a nutshell the photometer:
Measures each of the above variables: ambient temperature; ambient gas pressure;
the intensity of the UV light beam with
and without O
3
present;
Inserts know values for the length of the absorption path and the absorption
coefficient, and:
Calculates the concentration of O
3
present in the sample gas.
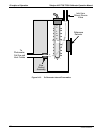
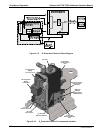
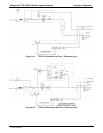
9.6.1.2. The Measurement / Reference Cycle
In order to solve the Beer-Lambert equation it is necessary to know the intensity of the
light passing through the absorption path both when O
3
is present and when it is not. A
valve called the measure/reference valve, physically located on front-left corner of the
O
3
generator assembly (see Figures 3-4 and 9-14) alternates the gas stream flowing to
the photometer between zero air (diluent gas) and the O
3
output from the O
3
generator.
This cycle takes about 6 seconds.
07223C DCN6572