Appendices
January 2009 UDA2182 Universal Dual Analyzer Product Manual 205
First Stage of Cyanide Destruction
Raise pH and oxidize cyanide
Sodium hydroxide (caustic) is used to raise the effluent to about 11 pH, which will
promote the oxidation reaction and ensure complete treatment. The oxidizing agent is
usually sodium hypochlorite, NaOCl. The reaction for the first stage is given below using
the NaOCl and with cyanide expressed in ionic form (CN
-
). The result is sodium cyanate
(NaCNO) and chloride ion (Cl
-
).
NaOCl CN NaCNO Cl+→ +
−−
This first-stage reaction is analyzed and controlled by independent control loops: caustic
addition by pH control and oxidizing-agent addition by ORP control (redox potential or
ORP, oxidation-reduction potential). Often an ON-OFF type of control using solenoid
valves or metering pumps can be used. The pH controller simply calls for more caustic
whenever pH falls below 11. The ORP controller calls for additional hypochlorite
whenever ORP potential falls below about +450 mV. (The metal ORP electrode is
positive with respect to the reference electrode.)
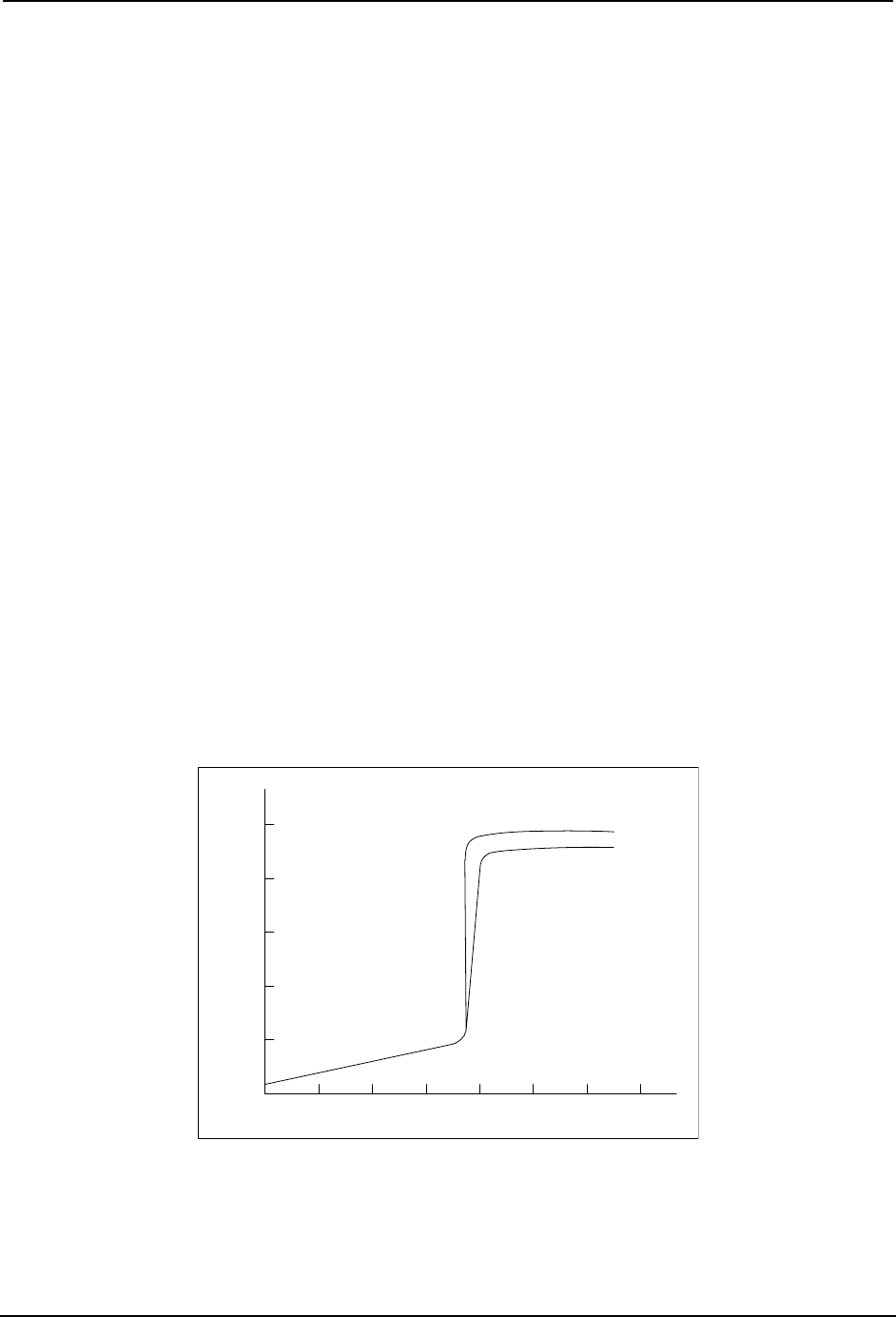
Titration curve
The ORP titration curve in Figure 15-4 shows the entire millivolt range if cyanide is
treated as a batch. For continuous treatment, operation is maintained in the oxidized,
positive region of the curve near the +450 mV setpoint. The ORP setpoint can vary
between installations, depending upon pH, the oxidizing agent, the presence of various
metals in solution, and the type of reference electrode used. Determine the exact setpoint
empirically at that potential where all the cyanide has been oxidized without excess
hypochlorite feed. This point can be verified with a sensitive colorimetric test kit or
similar check for cyanide.
VOLUME OF HYPOCHLORITE ADDED
REDOX POTENTIAL (mV)
0
1
2
3
4
5
6
7
-400
-200
0
+200
+400
+600
pH= 10.5
pH= 11.0
Figure 15-4 First Stage Cyanide Oxidation - Typical Titration Curve


















