Model T101 Instruction Manual Principles Of Operation
245
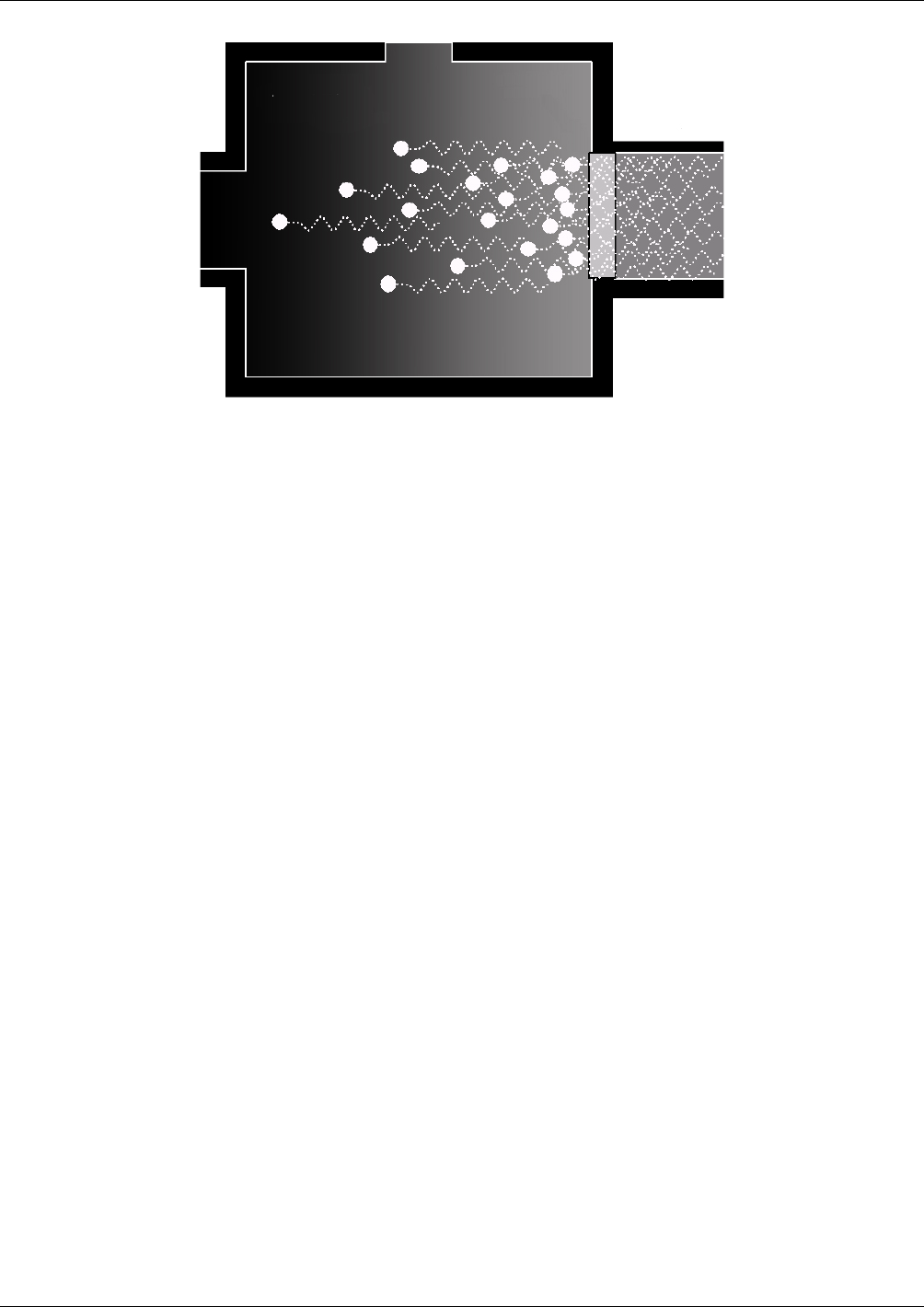
Figure 10-1. UV Absorption
The equation for defining the average intensity of the UV light (Ia) is:
20
SOaxexp1IIa
Where: (Equation 10-2)
I
0
= Intensity of the excitation UV light.
a
= The absorption coefficient of SO
2
.
SO
2
= Concentration of SO
2
in the sample chamber.
x
= The distance between the UV source and the SO
2
molecule(s) being
affected (path length).
The second stage of this reaction occurs after the SO
2
reaches its excited state (SO
2
*).
Because the system will seek the lowest available stable energy state, the SO
2
* molecule
quickly returns to its ground state (Equation 10-3) by giving off the excess energy in the
form of a photon (h). The wavelength of this fluoresced light is also in the ultraviolet
band but at a longer (lower energy) wavelength centered at 330nm.
nm33022
hvSO*SO
(Equation 10-3)
The amount of detectable UV (F) given off by the decay of the SO
2
* is affected by the rate
at which this reaction occurs (k).
07266B DCN6485


















